Chimeric antigen receptor, or CAR T-cell therapy, is a new form of immunotherapy that uses specially altered T cells to more specifically target cancer cells. The immune system is made up of specific cells and organs that protect the body from infection and cancer. Among these are T cells, which hunt down and destroy abnormal cells, including cancer cells. Sometimes, cancer cells find ways to evade the immune system; so, the immune system needs to be retrained to recognize and attack cancer cells. CAR T-cell therapy is one innovative approach to program the immune system to attack cancer.
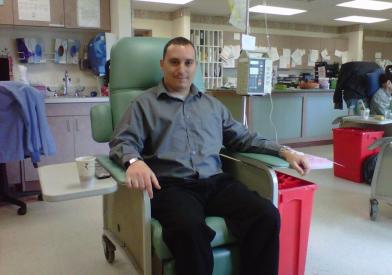
After a sample of a patient's T cells has been collected from the blood, the cells are re-engineered so they sprout special structures called chimeric antigen receptors (CARs) on their surface. When these CAR T cells are reinjected into the patient, the receptors may help the T cells identify and attack cancer cells throughout the body.
Currently, CAR T-cell therapy is FDA-approved as the standard of care for some forms of relapsed or refractory non-Hodgkin lymphoma, multiple myeloma, and adult and pediatric relapsed B-cell acute lymphoblastic leukemia (ALL), and is available through clinical trials for other forms of blood cancer.