Over the last decade, the Center for Chronic Lymphocytic Leukemia (CLL) at Dana-Farber Cancer Institute has been a leader in the development of novel therapies for patients with CLL, making major contributions to the initial approvals of several new drugs, including venetoclax (targeting BCL-2), ibrutinib, and acalabrutinib (targeting BTK), and idelalisib and duvelisib (targeting PI3K). Each of these drugs on its own has led to substantial improvements in the outcomes for patients with CLL, particularly those with high-risk disease markers; however, despite these major advances, CLL generally remains an incurable disease. Therefore, ongoing research in the Center is aimed at developing even better therapeutic approaches for this disease, largely through rationally designed novel agent combination regimens.
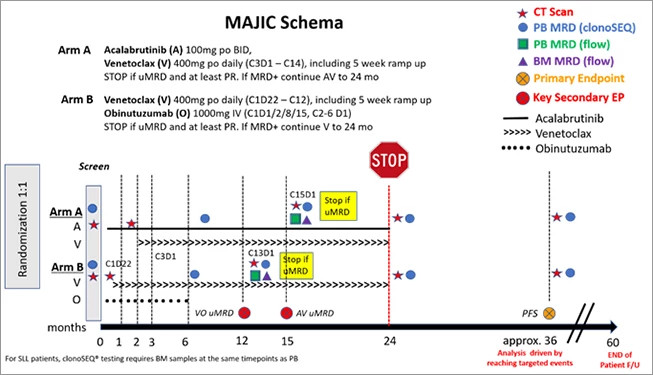
Dana-Farber's Division of Lymphoma Clinical Research Director Matthew Davids, MD, MMSc, generated laboratory data in collaboration with Anthony Letai MD, PhD, suggesting that dual targeting of BTK and Bcl-2 could be a highly effective strategy to treat CLL. Based on those initial studies, Dr. Davids developed an investigator-initiated, phase 2 clinical trial combining acalabrutinib, venetoclax, and obinutuzumab (the AVO trial) for patients with previously untreated CLL. This combination was very well-tolerated and led to undetectable minimal residual disease (MRD) in the bone marrow in 86% of patients. (Figure 1) Given these promising results, which the group recently published in Lancet Oncology, they have now opened an expansion cohort of the study focused on high risk patients with previously untreated, TP53 mutated or deletion 17p CLL. The AVO trial also led to the development of an exciting phase 3 study called MAJIC that we will open at Dana-Farber in late 2022. (Figure 2) This international study, on which Dr. Davids serves as co-PI, will compare acalabrutinib plus venetoclax to obinutuzumab plus venetoclax in a randomized fashion, and may lead to a new standard of care for frontline CLL treatment.
For patients with CLL who have already received treatment and subsequently progressed, the Center also has several promising new trials.
Another important recent development in the Center is the addition of an outstanding new faculty member Inhye Ahn, MD, who joined the group after several years on faculty at National Institutes of Health. In addition to providing highly expert care for CLL patients, Dr. Ahn is developing a broad research portfolio, including working to bring early intervention studies to patients with CLL who are on a watch and wait approach. One such study is called CLL NeoVax, which is exploring a personalized neoantigen vaccination strategy in higher risk CLL patients on watch and wait. This cutting-edge approach is based on elegant science developed in the laboratory of Catherine Wu, MD, Chief, Division of Stem Cell Transplantation and Cellular Therapies. Dr. Ahn is also working to bring a venetoclax-based early intervention study to Dana-Farber later this year.
Despite all the progress in CLL therapy over the last decade, many unanswered questions remain in this field, and thus much work still needs to be done to optimize outcomes for patients. With all of these studies, and many more in the portfolio, the Dana-Farber CLL Center strives to have an exciting clinical trial option available for patients at all stages of the disease, both to provide top notch care for current patients and to learn how best to treat the patients of the future.
- One such study that recently opened is an investigator-initiated phase 2 trial being led by CLL Center Director Jennifer Brown, MD, PhD, which combines a highly specific, well-tolerated BTK inhibitor zanubrutinib with venetoclax.
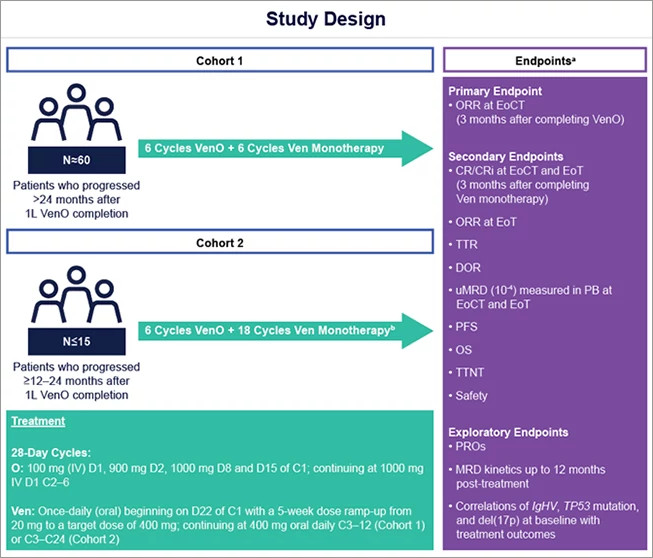
- A new study opening soon at Dana-Farber will answer another key question in the field. Specifically, since the frontline venetoclax plus obinutuzumab regimen is given as a one year, time limited therapy, it is unknown how effective this same regimen could be when used a second time in patients who achieved initial remission but subsequently relapsed. Dr. Davids will be leading an international study of retreatment with venetoclax and obinutuzumab (a drug formerly called GA-101), a trial that has been nicknamed the "ReVenG study" for "Re-treatment with Ven-G." Patients will be eligible if they were treated initially with venetoclax plus obinutuzumab and achieved at least one year of remission. (Figure 3)
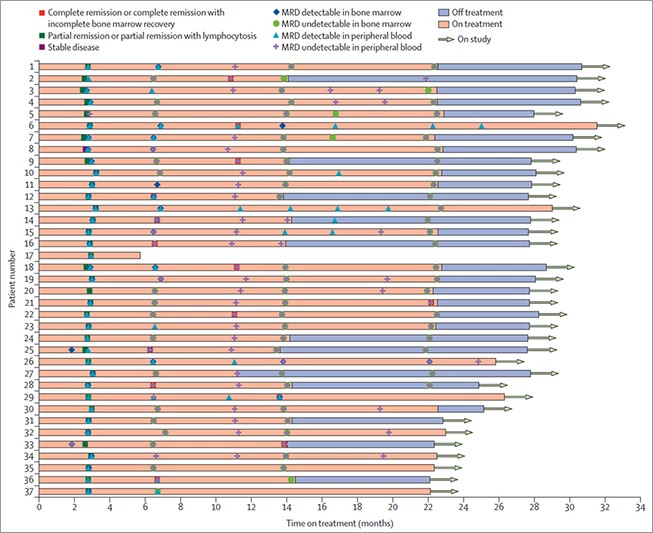
The timepoint at which each patient first reached the indicated response is shown. MRD = minimal residual disease.