Clonal hematopoiesis of indeterminate potential (CHIP) and clonal cytopenia of undetermined significance (CCUS) are characterized by the presence of acquired mutations in genes associated with myeloid malignancies at a variant allele fraction (VAF) of 2% or greater, in the absence and presence of cytopenia, respectively.1 CHIP and CCUS are prevalent precursors of myeloid malignancies, detectable in an estimated 10-20% of adults aged 70 and older.2-6 While the relative risk of myeloid malignancy is increased in CHIP/CCUS, the absolute risk is only 0.5 to 1% per year.2,3,7 CHIP/CCUS is 100-fold more prevalent than myeloid malignancies such as acute myeloid leukemia (AML),8 consistent with the knowledge that most individuals with CHIP/CCUS will not progress to a bona fide blood cancer. In recent years, hematology clinics specializing in CHIP/CCUS have been created10,11 to manage the growing number of patients with CHIP/CCUS mutations identified by next-generation sequencing (NGS) during the evaluation of solid tumors, workup of unexplained cytopenias, and diagnosis of potential germline cancer predisposition syndromes. The Clonal Hematopoiesis Risk Score (CHRS) addresses a major need for this population: estimation of the risk of myeloid malignancy.9
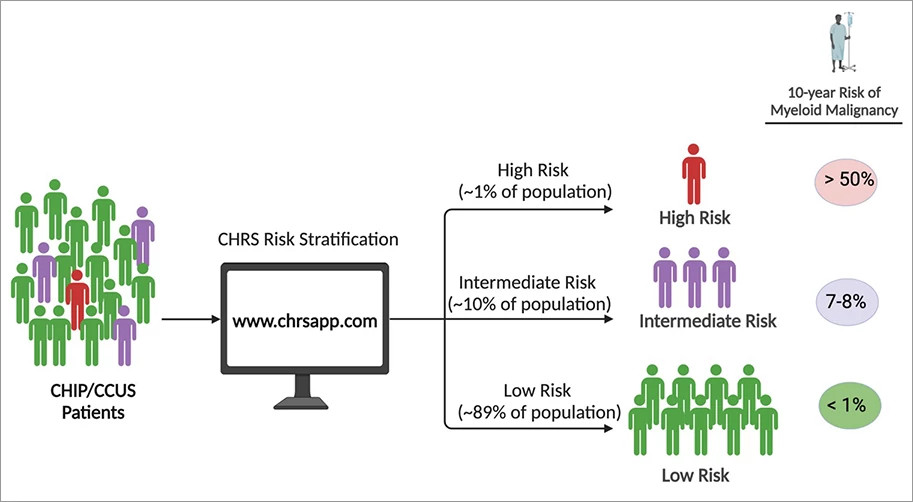
To derive the CHRS (available at www.chrsapp.com), we leveraged the genomic data linked to 10+ years of clinical outcomes for ~500,000 healthy volunteers enrolled in UK Biobank (UKB) study. We analyzed a discovery cohort of 11,337 individuals with CHIP/CCUS from UKB to identify and weight variables prognostic for progression to overt myeloid malignancy. Variables identified included the presence of/absence of the following: high risk mutations (mutations in splicing factor genes, IDH1/2, FLT3, RUNX1, or TP53), DNMT3A mutation as the only genetic abnormality, large clone size (VAF ≥ 20%), multiple mutations, increased red blood cell indices indicated by MCV ≥ 100fl or RDW ≥ 15%, cytopenia (CCUS vs CHIP), and age ≥ 65 years. We used multivariable regression to assign statistical weights to these prognostic variables. The CHRS score is determined by the sum of the statistical weights and is used to define three risk groups. At 10 years, the risk of myeloid malignancy was ≥ 50% in high-risk CHIP/CCUS (CHRS score ≥ 12.5), 7-8% in intermediate risk CHIP/CCUS (CHRS score 10-12), and < 1% in low-risk CHIP/CCUS (CHRS score ≤ 9.5).9 Most individuals were low-risk (88%) and only a small minority were high-risk (1.1%). We validated the CHRS using an additional cohort of 16,274 individuals with CHIP/CCUS from UKB, 646 CHIP/CCUS patients from Dana-Farber Cancer Institute, and 99 CCUS patients from the University of Pavia, Italy.
The CHRS is a clinically useful tool for myeloid malignancy risk estimation in CHIP/CCUS patients. The risk estimates provided by the CHRS help to counsel patients and rationally guide surveillance strategy. High-risk patients, particularly those with cytopenias, may benefit from more frequent monitoring of blood counts (e.g., every 3 to 6 months), clone composition (e.g. repeat sequencing every 1 to 3 years and with CBC changes) and bone marrow morphology (e.g. repeat bone marrow with changes in blood count, clone composition). Alternatively, less frequent monitoring of blood counts and clone composition of low- and intermediate-risk patients is warranted.
While several features of the CHRS overlap with those predictive of therapy-related myeloid malignancy in CHIP/CCUS,12 validation of the model is needed in solid tumor populations – a large volume of patients evaluated in specialized CHIP clinics within cancer centers.10,11 Additionally, given the importance of clonal selection12,13 and dynamics14 on progression, including data from multiple time points may allow for refinement and personalization of future iterations of the CHRS.
CHRS risk stratification is a major step towards developing early detection and prevention programs for myeloid malignancies. Ultimately, however, management of CHIP/CCUS will have public health relevance only if doing so prevents disease and improves overall survival. Numerous clinical trials testing therapeutic intervention in CCUS are underway or in development, with endpoints including hematologic improvement and incidence of hematologic malignancy.15 Using the CHRS to help define trial eligibility is one strategy to target interventions to the highest-risk patients, who are the most likely to derive any clinical benefit from therapeutic intervention. With luck, these studies will identify safe and effective strategies for early intervention to prevent myeloid malignancy in CHIP/CCUS.