Axicabtagene ciloleucel (axi-cel) is among three FDA-approved autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapies that have dramatically shifted the landscape of relapsed/refractory large B-cell lymphoma (LBCL) management in the post second-line setting. The seminal ZUMA-1 study demonstrated axi-cel's ability to induce a robust 82% overall response rate (ORR), with 54% achieving complete responses (CR) among heavily pretreated patients1. The efficacy data generated from this registration trial resulted in axi-cel's regulatory approval in 2017, and two additional anti-CD19 CAR T cell approvals for the same indication have followed since 2,3. Longer-term follow up from the ZUMA-1 population suggests that not only do CAR T cells have the capacity to induce high response rates, but they also confer durable long-term remissions in over half of responsive patients at five years 4.
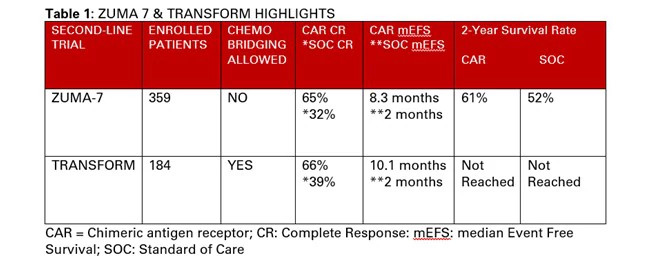
Given this notable efficacy of cellular immunotherapy in later-line settings, the phase 3 ZUMA-7 trial, site led by Dana-Farber Cancer Institute's Caron Jacobson, MD, MMSc, explored axi-cel in the second-line setting vs. standard-of-care (SOC) therapy5. This study enrolled 359 autologous stem cell transplant (ASCT)-eligible patients with LBCL who relapsed within one year of first-line chemoimmunotherapy. Participants were randomized to axi-cel vs. platinum-based salvage chemotherapy followed by an ASCT for responders (non-responders were treated off-study). Of the 140 patients assigned to axi-cel, 94% were infused, whereas only 64 of the 179 patients (36%) randomized to SOC received a destination transplant due to treatment refractory disease.
Axi-cel treated patients achieved an ORR of 83% and CR rate of 65% compared to respective rates of 50% and 32% among those receiving SOC. At a median follow-up mark of 25 months, axi-cel treated patients achieved a higher median event-free survival (EFS) of 8.3 months vs. 2 months (p < 0.0001). Additionally, median overall survival was not reached among axi-cel recipients vs. 35 months in the control group (P=. 027).
Albeit with a shorter median follow-up of 6 months, similar results were achieved in the concomitantly reported phase 3 TRANSFORM trial that examined the potential benefit of lisocabtagene maraleucel (liso-cel) in the second-line setting as mentioned above 6. Although with a slightly different study design permitting bridging chemotherapy, liso-cel treated patients achieved a higher CR rate of 66% (vs. 39% in control) and experienced a superior median EFS of 10.1 months (vs. 2.3 months in control) among 184 enrolled patients. Additionally, 54% (50 out of 92) of patients assigned to the SOC crossed over to receive liso-cel, which is in keeping with the ASCT ineligibility rates noted in ZUMA-7.
Overall, the incidence of cytokine release syndrome and neurotoxicity with axi-cel and liso-cel were comparable to previously reported rates in other trials without any fatal events.
Collectively, these two studies highlight the therapeutic superiority of liso-cel and axi-cel in the second-line setting. While there were some differences in trial design across studies, the data reliably confirm an unmet need among the high proportion of patients who exhibit chemo-recalcitrant disease, where ASCT is known to offer little to no benefit. It is notable, however, that the optimal salvage strategy among patients who relapse beyond one year after first-line induction remains to be investigated. Nevertheless, as we await longer follow-up data to establish the therapeutic reliability with this approach, the supplantation of second-line ASCT by axi-cel and liso-cel in management of chemo-refractory LBCL is quickly gaining momentum.